Investigation Launched into Botox Injections Linked to Botulism Outbreak
Federal officials are currently investigating the origin of Botox injections, some of which may be counterfeit, that have been associated with a series of botulism-like illnesses across various states in the U.S.
The Food and Drug Administration (FDA) is collaborating with other federal and state agencies to pinpoint the cause of the outbreak that has affected a minimum of six individuals in Illinois and Tennessee who received injections containing the botulinum toxin.
In Tennessee, the Department of Health reported that four individuals in the state exhibited symptoms resembling botulism, with two of them requiring hospitalization after receiving potentially counterfeit injections.
Meanwhile, in Illinois, health authorities are cautioning medical practitioners to remain vigilant for patients displaying signs of botulism following two cases where individuals experienced symptoms such as blurred vision, facial drooping, and breathing difficulties. Both affected individuals received injections from a licensed nurse in LaSalle County who administered the treatment without proper authorization.
The Centers for Disease Control and Prevention (CDC) noted that the botulinum toxin injections, commonly known as Botox, were administered in "non-medical" environments, and the origins of these products are currently unidentified or unverified.
Allergan, the official manufacturer of FDA-approved Botox, has not yet provided a response to requests for comments regarding the situation.
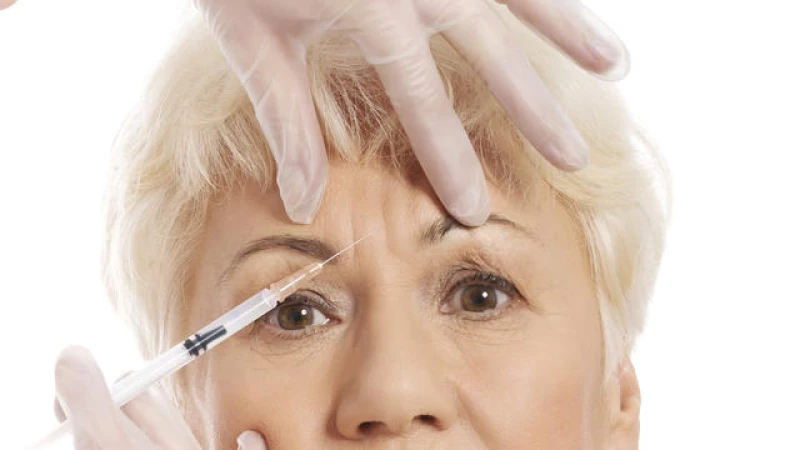
Approved for cosmetic use more than 20 years ago, Botox is a popular drug used to smooth wrinkles and look younger, with injections typically costing around $530, according to the American Society of Plastic Surgeons. The effects of a shot last three to four months on average, so additional shots are needed to remain wrinkle-free.
Botulism is a serious and sometimes fatal illness caused by a toxin that can be transmitted by food or result from untreated wounds, while infants can develop an intestinal form of the illness, according to the CDC.
So-called iatrogenic botulism is caused by excessive exposure to the botulinum toxin, although confirmed cases occurring after cosmetic or therapeutic injections are rare, according to health officials. Injections should involve an FDA-approved product administered by a licensed provider, health experts advise.
The FDA urged people experiencing adverse effects or health care providers receiving patients with adverse effects to report them .
Federal officials have previously cracked down on unregulated Botox and other cosmetic treatments. In 2023, U.S. Customs and Border Protection officers in Ohio intercepted such fillers that had been shipped from Bulgaria, China, Korea, and Spain.