Heart Transplant Device Leads to Patient's Death
Arvid Herrman, a 68-year-old Wisconsin farmer, faced a difficult choice when he was deemed too old and too sick for a heart transplant. He could either have a mechanical pump, known as a HeartMate 3, implanted in his heart to potentially extend his life, or he could opt for no treatment and face certain death within a year.
Choosing the mechanical pump, which is currently the only FDA-approved device of its kind, Herrman hoped for a chance at a longer life. However, tragedy struck when a defect in the locking mechanism of the HeartMate 3 prevented the device from sealing properly. This led to multiple strokes, a severe brain injury, and ultimately, multiorgan failure, resulting in Herrman's death.

The lawsuit filed by Herrman's daughter, Jamie Edwards, alleges that her father's death was caused by the defect in the HeartMate 3. It states that Herrman "could not have anticipated the danger this defect... created for him."
The incident has been reported to the Food and Drug Administration, and the details of the case can be found in the FDA's public database of device-related deaths, serious injuries, and malfunctions. Additionally, the event has been documented in the peer-reviewed Journal of Heart and Lung Transplantation.
In September 2021, a patient named Ramon Flores Sr. underwent a surgical procedure at Methodist Hospital of San Antonio, during which he had a device implanted. Tragically, just eight days after the surgery, Flores passed away at the age of 76. His family filed a lawsuit in August, alleging that a defect in the device's locking mechanism resulted in air embolism strokes, ultimately leading to his death.
According to Flores' daughter, Alanna Flores Blanco, the family was never informed about the possibility of device malfunction and the potential risks associated with it. She expressed concern about how many others might be affected by similar incidents.
Following the deaths of Herrman and Flores, the manufacturer of the device, Thoratec Corp., conducted an evaluation of the pumps involved. However, Abbott, the manufacturer, did not respond to inquiries regarding the deaths or the alleged defects. The manufacturer has denied liability in both cases. While Herrman's lawsuit was settled earlier this year, the Flores case remains ongoing.
According to an analysis conducted by KFF Health News, there have been over 4,500 reports since August 2017 suggesting that the HeartMate 3 device may have caused or contributed to patient deaths. These findings were based on the FDA's database of medical device incidents, known as the Manufacturer and User Facility Device Experience (MAUDE). The database collects reports of device-related deaths, serious injuries, and malfunctions from hospitals, doctors, and other sources. Manufacturers are obligated to investigate such incidents.
Breakthrough Heart Pump Device Shows Promising Results
A recent study has shown that the HeartMate 3, a left ventricular assist device (LVAD), has significantly improved the safety and effectiveness of mechanical heart pumps. The HeartMate 3, which was first approved by the FDA in 2017, has been hailed as the safest iteration of LVADs to date.
Patients with end-stage heart failure often turn to LVADs as a last resort, and the complexity of the device combined with the patients' comorbidities creates dynamic clinical care situations. However, the HeartMate 3 has addressed many of the complications that have historically been associated with heart pump technology, such as clotting, stroke, and bleeding.
Dr. Jonathan Paquette, a cardiologist at the University of Chicago Medicine, emphasized the significance of this breakthrough, stating, "The HeartMate 3 has dramatically improved the safety of LVADs. It has reduced rates of complications that have historically challenged heart pump technology."
The HeartMate 3 is a long-term therapy option for patients awaiting a heart transplant. It has shown promising results in improving patient outcomes and quality of life.
Recently, the FDA expressed its support for the device, HeartMate 3, stating that the benefits outweigh the risks for the patient population that has limited alternatives available. Jeremy Kahn, a spokesperson for the agency, made this statement in August.
Larry Kessler, the founder of Device Events, expressed concern about the safety of the device. He believes that this is a safety signal, and he is unsure if the FDA is taking any action to address it.
For more information, you can visit the website of Device Events: https://deviceevents.com/
You can also learn more about Larry Kessler by visiting his profile on the University of Washington website: https://sph.washington.edu/faculty/facbio/Kessler_Larry
Patients and their caregivers are often left uninformed about the risks associated with medical devices like the HeartMate 3 due to limitations in the information available, according to Sanket Dhruva, a cardiologist and expert in medical device safety and regulation at the University of California-San Francisco.
Dhruva emphasizes that patients facing the decision of whether or not to proceed with a left ventricular assist device (LVAD) are left with incomplete data and uncertainty about the risks they may face. This lack of information makes it difficult for doctors to effectively counsel patients using the FDA database as a tool.
Dhruva points out that if doctors cannot distinguish between real safety signals and irrelevant information in the database, it becomes challenging to have meaningful discussions with patients about the benefits and risks of medical devices.
Dhruva also highlights that the HeartMate 3 is not the only device with a difficult-to-determine safety profile in the FDA database. The information available in the database is insufficient to provide patients with a comprehensive understanding of the safety risks associated with any medical device. This reflects the overall weakness of postmarket surveillance after a device has been approved for sale.
Under federal regulations, device manufacturers typically must report adverse events to the FDA within 30 days of learning about them, and that data is often used by researchers and regulators to identify potential safety concerns. Reports also can be submitted voluntarily by doctors, patients, or others. The FDA says that reports don't need to be filed if the manufacturer determines that a device did not cause or contribute to an adverse event.
"Health care facilities, and risk managers in particular, they aren't always forthcoming with detailed data about events," he said.
Reports in MAUDE show that patients with a HeartMate 3 have experienced adverse events, such as bleeding, infection, and respiratory failure, that the manufacturer warned were possible in its instructions for use.
Reports in MAUDE also describe fatal incidents due to complications not mentioned in the manufacturer's instructions, such as the locking mechanism malfunction. In one report, a patient died of smoke inhalation after an external battery charger caught fire.
Flores Blanco was completely unaware of MAUDE prior to her father's surgery. Even if she had known about it, it would have been highly unlikely for her to discover a problem with the locking mechanism amidst the overwhelming amount of records, let alone foresee the potential consequences.
Communication breakdown?
During their visit, FDA inspectors did not identify any deficiencies in how Thoratec handled complaints. According to records, the company had received a staggering 8,115 complaints related to the HeartMate 3 within the 12 months leading up to the inspection in October 2020.

In the perspective of Kinard, device-makers generally exceed the 30-day timeframe when investigating the underlying cause of an incident. They often attribute adverse events to user error in order to downplay the device's problems," she stated.
The lawsuit states that Herrman's surgeon, John Stulak, who was involved in the clinical trial that introduced the HeartMate 3 to the market, has extensive experience in implanting the device. However, Stulak did not respond to interview requests. In a publication in The Journal of Heart and Lung Transplantation in 2020, Stulak and two colleagues from the Mayo Clinic detailed Herrman's case, highlighting the malfunction of the locking mechanism. They wrote, "The lack of a tight seal from this defect resulted in multiple subsequent air embolism events and irrecoverable neurological damage."
Two individuals, Herrman and Flores, have tragically passed away due to different medical conditions.
Herrman's death certificate states that he suffered complications from ischemic heart disease.
On the other hand, Flores' death certificate indicates that he died from cardiac arrest and hypoxic ischemic encephalopathy, which is a form of brain damage.
Even the FDA has encountered challenges in maintaining the accuracy and timeliness of the MAUDE database.
A last-resort treatment
Before receiving a HeartMate 3 implant in January 2022, Sid Covington, a resident of Austin, Texas, extensively researched the device after years of medication therapy and cardiac rehabilitation to manage his congestive heart failure.
"I examined case studies. I reviewed various heart studies," Covington stated. "I even went through their marketing brochures and anything else I could find."
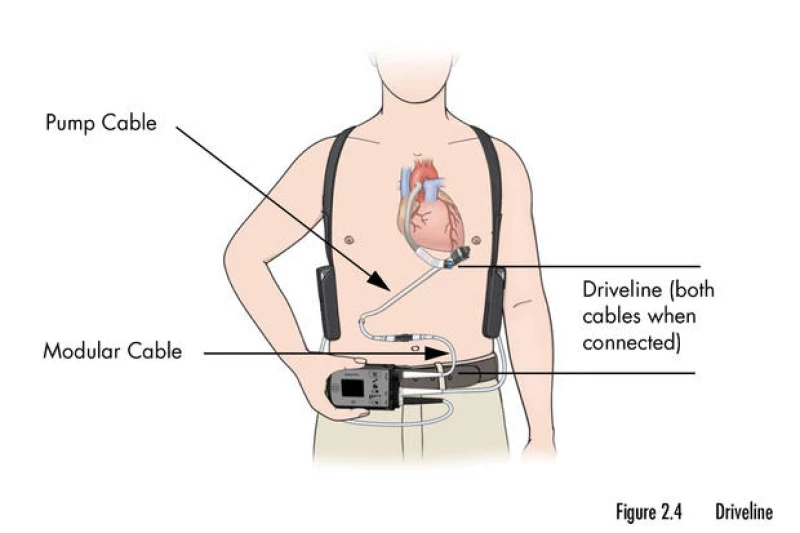
Covington, who is 76 years old, admitted to being aware of MAUDE and Intermacs, a private registry that tracks LVAD patients, but did not seek their consultation. When faced with the decision of whether to receive the device, he was hospitalized due to chest pain, shortness of breath, and fatigue resulting from advanced heart failure. Covington claimed that the HeartMate 3 was his only option.
"When it comes down to the moment, you really don't have much choice," he explained. "It's any port in the storm at that point."
Patients who receive the HeartMate 3 device for end-stage heart failure must take constant precautions to ensure its proper functioning. They are advised to keep the external parts of the device dry at all times and avoid activities such as jumping and contact sports. Additionally, patients need to ensure that the device always has a source of power, which is supplied through a cord attached to the pump that exits the body through a surgical opening.
According to Larry Allen, a cardiologist from the University of Colorado, patients who opt for the HeartMate 3 device are often left with no other treatment options for their heart failure. The decision to proceed with the device is only made when the risk of death is deemed extremely high and all other alternatives have been exhausted.

The regulatory view on high-risk medical devices, such as the HeartMate 3, takes into consideration the severity of the patient's condition. According to Kessler, the FDA is willing to accept a potentially higher risk for seriously ill patients, as long as it is not deemed irresponsible and can be effectively communicated to clinicians and the public.
Allen, who has contributed to the development of a decision aid for patients considering the HeartMate 3 device, emphasizes the importance of reliable data on safety and risks. He believes that the decision to undergo this treatment is complex and requires more comprehensive informed consent processes.
Data exists but Is confidential
Long-term data for the HeartMate 3 — including performance metrics for the more than 180 U.S. hospitals certified to implant the device — are kept in Intermacs, managed by The Society of Thoracic Surgeons, which has promised to provide transparency but has yet to deliver.
The registry tracks mortality and injury rates for patients with an LVAD and logs the number of devices implanted each year.
But Intermacs is proprietary, and access at hospitals requires a principal investigator and at least one trained staff member, who can use the data to evaluate their facility's performance against an aggregate from their peers across the nation.
Francis Pagani, a heart transplant and LVAD surgeon at University of Michigan Health, leads a medical society task force that oversees Intermacs. He said 12,000 to 14,000 HeartMate 3 implants have been recorded in Intermacs since 2017. The HeartMate 3 has "the best outcomes of any other LVAD, ever," he said.
Over the years, federal regulators have made it easier for patients to access LVADs, reducing surgery volume requirements for implant centers and no longer requiring patients to be on a transplant waiting list to receive one of the pumps.
Though the HeartMate 3 is presently the only LVAD being implanted in the United States, it once had a competitor, Medtronic's HeartWare, which the manufacturer removed from the market in June 2021, citing a high risk of stroke and pumps failing to restart if stopped.
While the FDA provides consumers with concise information about key clinical trials supporting the approval of new drugs, the agency provides no comparable data for medical devices. And though Medicare reimburses hospitals nearly $200,000 for most HeartMate 3 implants, federal administrators do not track patient outcomes or enforce performance standards for the heart pumps.
James Kirklin, a cardiac surgeon and researcher, was the principal investigator for Intermacs when the FDA, Centers for Medicare & Medicaid Services, and National Heart, Lung, and Blood Institute awarded a contract to the University of Alabama at Birmingham to establish the registry in 2005.
Federal agencies paid about $15 million over 10 years for Intermacs, Kirklin said, because they wanted to better understand the risk factors for death and other adverse events with so-called mechanical circulatory support devices, including LVADs, as well as the factors that indicated a higher likelihood of patients doing well on the pumps.
Kirklin is collaborating with The Society of Thoracic Surgeons to develop a risk model that will allow the public to view quality scores for hospitals that perform LVAD implantations. This has been recognized as a necessary tool since at least 2018. However, it will take approximately a year before the model is completed.
"When there is a high number of deaths, it prompts an investigation. I completely agree," Kirklin stated. "But the information is limited. It does not consider time and the denominator is unknown. If you look up Intermacs, all the data is available."
The families of Herrman and Flores have filed lawsuits in order to determine what went wrong. Herrman's family has settled the lawsuit and agreed to keep the details confidential. Thoratec has filed a motion to dismiss the ongoing Flores case based on the FDA's approval of the device.
Alanna Flores Blanco mentioned that both she and her father were aware of the positive outcomes associated with the HeartMate 3, including published research indicating a more than 50% chance of living for five years or longer for those who receive the device.
"That's why he decided to go through with it," she explained.
Flores Blanco's father was an exemplary patient, diligently following the advice of his cardiologists and other specialists. He attended classes to learn how to live with his medical device and even received approval for surgery from the medical review board at Methodist Hospital in San Antonio.
The family believed that they were well-informed and that her father was adequately prepared for the surgery.
"He did everything he was supposed to do," Flores Blanco said. "Unfortunately, it was the device itself that let him down."
KFF Health News, formerly known as Kaiser Health News, is a national newsroom that produces in-depth journalism about health issues. It is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.







